Hepatitis
B Virus Genotype G Associated with Increased Fibrosis in HIV/HBV Coinfected
People, but Blacks Fare Better
 |
 |
 |
 |
 |
 |
 |
| SUMMARY:
Hepatitis B virus (HBV) genotype G, found in 17% of a Texas
cohort of HIV/HBV coinfected patients, was associated with
more aggressive liver fibrosis progression, according to a
poster presented at the 17th Conference on Retroviruses &
Opportunistic Infections (CROI 2010)
last week in San Francisco. Investigators also found that
African-Americans tended to have milder fibrosis, while Hispanics
were at greater risk of advanced liver disease. |
|
 |
 |
 |
 |
 |
 |
 |
By
Liz Highleyman
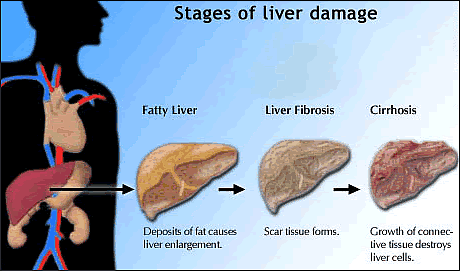
Over years
or decades, chronic hepatitis B can
lead to advanced liver disease, and some evidence indicates that this
may happen more rapidly in HIV/HBV
coinfected people. But the effect of HBV genotypes on disease progression
in this population is not fully understood.
Doan Dao from the University of Texas Southwestern Medical Center and
colleagues examined the prevalence and impact of genotype on liver
fibrosis in coinfected patients.
Genotype
G is a defective form of HBV that replicates poorly in most settings,
the investigators noted as background. Prior research indicated that
this genotype is present in less that 1% of HBV monoinfected people,
is hepatitis B "e" antigen (HBeAg) negative, and typically
results in low HBV DNA viral load.
Experiments
in immunocompromised animals suggest that HBV genotype G frequently
mixes with other genotypes to enhance its replication capacity, they
continued. The resulting mixed form causes cellular steatosis (fat accumulation)
and has direct cytopathic (cell-killing) effects on liver cells, thereby
promoting liver fibrosis.
The investigators determined HBV genotype(s) in stored serum samples
from 133 HIV positive individuals
with hepatitis B surface antigen (HBsAg) who received care at a Dallas
teaching hospital.
Most participants (84%) were men, the median age was 42 years, about
half were black, 38% were white, and 11% were Hispanic. About one-quarter
were positive for hepatitis C virus (HCV) as well as HIV and HBV. Most
(again, 84%) had absent or mild fibrosis at baseline. The median CD4
cell count was quite low, at 190 cells/mm3.
The researcher quantified HBV DNA levels and measured HBeAg and anti-HBe
antibody titers. They used the Fib-4 non-invasive biomarker index (which
includes ALT, AST, and platelet levels) to assess fibrosis stage. The
median follow-up time was 35 months (range 0 to 110 months).
Results
 |
The
HBV genotype distribution among the study participants was as follows: |
| |
 |
Genotype
A: 74%; |
 |
Genotype
G: 17%; |
 |
Genotypes
D: 5%; |
 |
Genotype
F: 1.5%; |
 |
Genotype
H: 1.5%. |
|
 |
Genotype
G appeared in mixtures with other HBV genotypes, confirmed by the
presence of high HBeAg levels (87%), HBeAg positive, undetectable
HBe antibodies (86%), and high HBV DNA levels (median 8.0 log10
copies/mL). |
 |
Genotype
G was associated with a significantly greater frequency of advanced
fibrosis (39%) compared with genotype A (1%). |
 |
African-American
patients had a significantly higher prevalence of mild fibrosis
compared with white or Hispanics patients. |
 |
In
a multivariate analysis, having HBV genotype G was associated with
increased fibrosis (odds ratio [OR] 4.3, or more than 4-fold higher
risk), while being African-American was associated with milder fibrosis
(OR 0.17). |
"HBV
genotype G was prevalent in the HIV-infected population but likely had
gained replicative fitness and HBeAg production by mixing with other
genotypes, most likely genotype A," the researchers concluded.
"HBV genotype G in the mixed form with other HBV genotypes would
explain the high HBV DNA and HBeAg levels observed.
"Despite
significantly shorter time of follow-up, patients with HBV genotype
G appear [to] have more advanced fibrosis [among] HIV/HBV coinfected
patients," they continued. "The reason for this more aggressive
disease is unclear."
Finally,
they recommended, "In HIV/HBV coinfection, HBV genotyping may be
an important tool to assess if the patient is at increased risk for
liver disease progression."
University of Texas Southwestern Medical Center, Dallas, TX.
2/26/10
Reference
D
Dao, J Balko, N Attar, and others. Genotype G Hepatitis B Virus (HBV)
and Advanced Liver Fibrosis in HIV/HBV Co-infected Patients. 17th Conference
on Retroviruses & Opportunistic Infections (CROI 2010). San Francisco.
February 16-19, 2010. (Abstract
626).